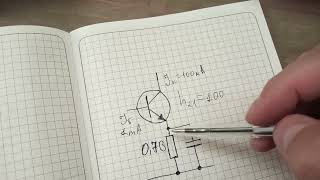
The Biomaterials and Biomolecules Facility is a Current Good Manufacturing Practices (CGMP) grade laboratory located in Rochester. CGMP refers to regulations enforced by the U.S. Food and Drug Administration (FDA) and provides for systems that assure proper design, monitoring and control of manufacturing processes and facilities. This type of laboratory environment permits first-in-human use of novel materials and devices, including implantable devices, tissue engineered scaffold, bio artificial organs and tendon repair lubricants.
Learn more: [ Ссылка ]
























































![AI Generated sci-fi future cities art - Technical Evolution - AI Generated Images [AI Generated 21]](https://s2.save4k.org/pic/Lc06NH_9GF0/mqdefault.jpg)