Problems:
1. A bag of ice was placed on a patient’s head. The ice bag contained 220.0g of ice at 0.00°C. When the ice bag was removed, all of the ice inside had melted and the liquid had a temperature of 21.00°C. How many joules of heat were added?
2. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C?
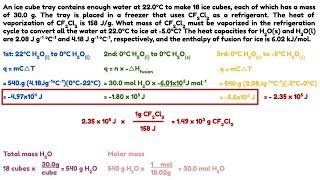
3. An ice cube tray contains enough water at 22.0oC to make 18 ice cubes, each of which has a mass of 30.0 g. The tray is placed in a freezer that uses CF2Cl2 as a refrigerant. The heat of vaporization of CF2Cl2 is 158 J/g. What mass of CF2Cl2 must be vaporized in the refrigeration cycle to convert all the water at 22.0oC to ice at -5.0oC? The heat capacities for H2O(s) and H2O(l) are 2.08 J g-1 oC-1 and 4.18 J g-1 oC-1, respectively, and the enthalpy of fusion for ice is 6.02 kJ/mol.
4. When ice at 0.0°C melts to liquid water at 0.0°C, it absorbs 0.334kJ of heat per gram. Suppose the heat needed to melt 35.0-g of ice is absorbed from the water contained in a glass. If this water has a mass of 0.210kg and a temperature of 21.0°C, what is the final temperature of the water? [Note that you will have 35.0 g of water at 0°C from the ice.
5. How many joules are required to convert 10.0g of solid ethyl alcohol at -180.3°C to the vapor state at the boiling point of 78.3°C? (C2H60)
a. C [solid EtOH] = 0.971J/g°C
b. C [liquid EtOH] = 2.30J/g°C
c. The melting point of alcohol is -117.3°C
d. ∆Hfus= 218J/g
e. ∆Hvap= 854 J/g



![[FULL SERMON] THE WEAPONS OF OUR WARFARE ARE NOT CARNAL (part 1) - Apostle Joshua Selman 2022](https://i.ytimg.com/vi/Aj8g6HxaK6o/mqdefault.jpg)





































































