Point defects in ceramics and polymers are more complex than those in metals. The reason for this is that ceramics constitute anion and cation. Since these ions are charged and electroneutrality must be maintained, then defects must come in pairs, rather than single defects. There are many types of ceramic defects. Two common ones are the Frenkel defect and Schottky defect. Frenkel defects are a vacancy paired with a self-interstitial. Schottky defects are a vacancy of both cation and anion atoms.
Polymers can be similarly complex. A point defect might be a foreign mer placed along the chain of a different polymer or a mer that is placed backwords with heat-tail configuration instead of tail-head etc.





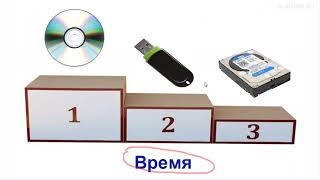






















































![Как работает Графика в Видеоиграх? [Branch Education на русском]](https://s2.save4k.org/pic/_j8R5vlA0ug/mqdefault.jpg)
![Как работает Клавиатура? [Branch Education на русском]](https://s2.save4k.org/pic/xCiFRXbJTo4/mqdefault.jpg)



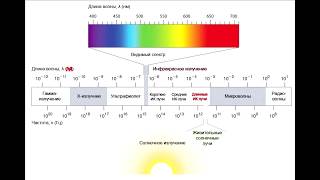






![Гелертер верят - Развитая цивилизация существовала до появления людей? [Времени не существует]](https://s2.save4k.org/pic/pMxzC99_ZkE/mqdefault.jpg)