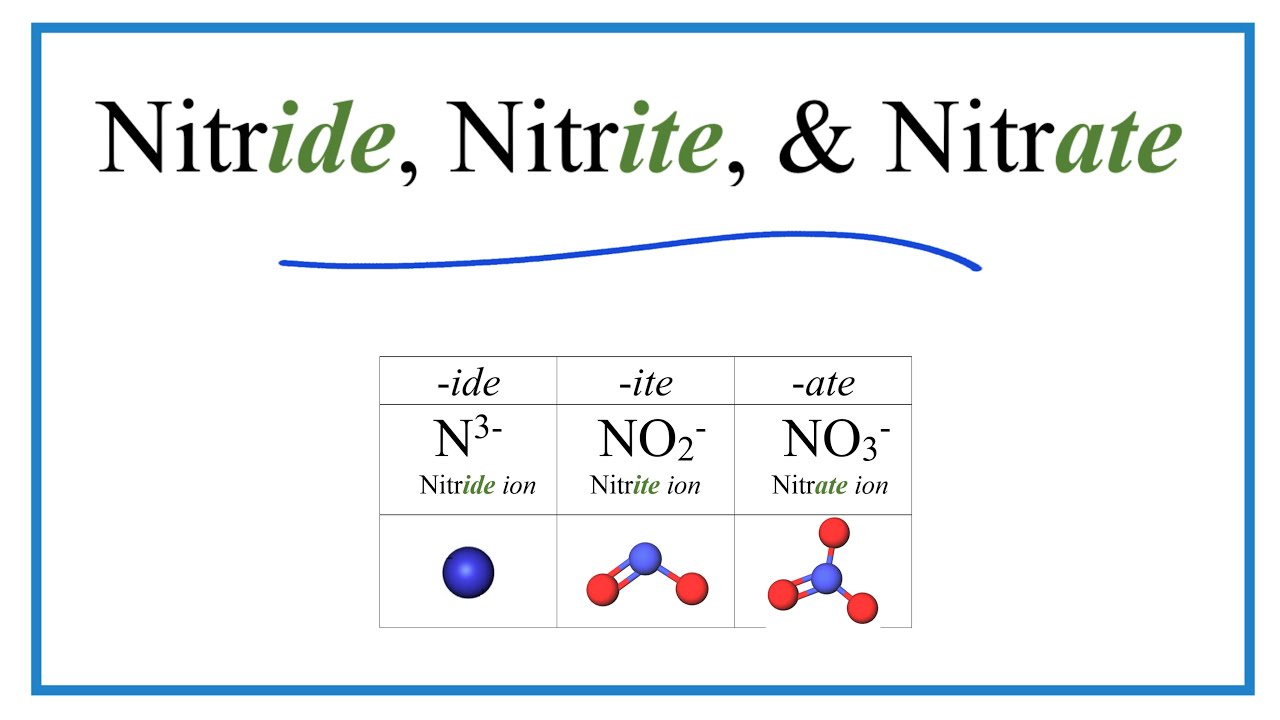
In chemistry it is important to be able to tell the difference between the Nitride, Nitrite, and Nitrate ion. While the names are similar, they are very different substances.
Nitride is the ion of Nitrogen. It's formula is N 3- .
Nitrite is a polyatomic ion with a 1- ionic charge. It's formula is NO2 - .
Nitrate is a polyatomic ion with a 1- ionic charge. It's formula is NO3 - .
It is probably a good idea to memorize the Nitrate ion (NO3 -) since it comes up frequently in chemistry. You can then use the naming rule that an ion ending in -ite has one less Oxygen atom that the ion ending in -ate. So if Nitrate is NO3 - , then Nitrite is NO2 -. This rule can be used with other similar polyatomic ions as well.
Helpful Videos
Memorizing the Polyatomic Ions: [ Ссылка ]
Finding Ionic Charge for Elements: [ Ссылка ]
Lewis Structure for Nitride ion: [ Ссылка ]
Lewis Structure for the Nitrite ion: [ Ссылка ]
Lewis Structure for the Nitrate ion: [ Ссылка ]
More chemistry help at http:/www.Breslyn.org