Brooke Harmon, Sandia National Laboratories
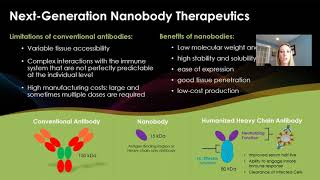
Monoclonal antibodies are popular therapeutics due to their favorable safety, target specificity, and pharmacokinetics compared with traditional small-molecule drugs. Several therapeutic antibody candidates effective against emerging viruses have been recently identified, but there are a number of caveats associated with conventional antibodies as therapeutics such as inadequate pharmacokinetics and tissue accessibility, impaired interactions with the immune system, sensitivity to viral escape, high manufacturing costs and multiple doses required before and after exposure. At Sandia National Laboratories we have developed technology platforms that can be used individually or in combination to address these challenges. These platforms use a Sandia designed proprietary next generation synthetic nanobody phage display library to swiftly identify antibody fragments with high binding affinity for any target of interest. The extreme diversity of our synthetic humanized nanobody library (3.24 x 1010), enabled by novel DNA synthesis techniques, makes this is a robust method for novel therapeutic discovery. With this library, highly sensitive and antigen specific nanobodies can be identified and validated within 4 months. Nanobodies are the target binding region of single-domain heavy chain only antibodies (sdAbs), derived from camelids. In comparison to conventional antibodies, nanobodies and sdAbs are smaller, more stable, and hydrophilic making them cheaper and easier to manufacture and produce at large scale. Due to their hydrophilic surface, multiple nanobodies with different targets can be easily linked without any mispairing issues, leading to an increase in binding affinity and resistance to viral escape mutants. Nanobodies and sdAbs are transported more efficiently into the blood and have better tissue penetration than conventional antibodies. To further improve pharmacokinetics, we have developed a functional and selective screening platform, using the same high diversity nanobody library, to identify tissue targeting moieties that can be conjugated to nanobodies, sdAbs, conventional antibodies, or any delivery vehicle to increase concentrations of therapeutics at the site of infection reducing the dose required to achieve clinical results. As a final enhancement strategy, we have developed tools to modulate antibody-triggered effector functions through engineered antibody constant (Fc) domains, to mediate optimal therapeutic effects. Pathogen specific nanobodies can be fused to human Fc domain with the appropriate modifications to make enhanced humanized sdAbs with all the attributes of nanobodies with improved half-life and optimized effector functions. By controlling tissue distribution, increasing neutralization potency, leveraging the ability of antibodies to engage innate immune cells to clear infected cells, and tissue targeting, multivalent nanobody-based antibody therapeutics have significant therapeutic, manufacturing and cost advantages over conventional antibody therapeutics and existing bispecific antibody formats.