Presented By: Kayvon Modjarrad, MD, PhD
Speaker Biography: Kayvon Modjarrad, MD, PhD is the founding Director of the Emerging Infectious Diseases Branch at the Walter Reed Army Institute of Research and Associate Professor of Medicine at the Uniformed Services University. Dr. Modjarrad leads the U.S. Army's development of a vaccine against COVID-19, co-leads all of WRAIR’s COVID-19 response efforts and serves as a member of Operation Warp Speed. Dr. Modjarrad is also an advisory member of the World Health Organization’s Strategic Advisory Group of Experts Immunization Working Group on COVID-19 Vaccines and Vaccination.
Webinar: Keynote Presentation: Toward Universal Coronavirus Vaccines: From Concept to Clinic
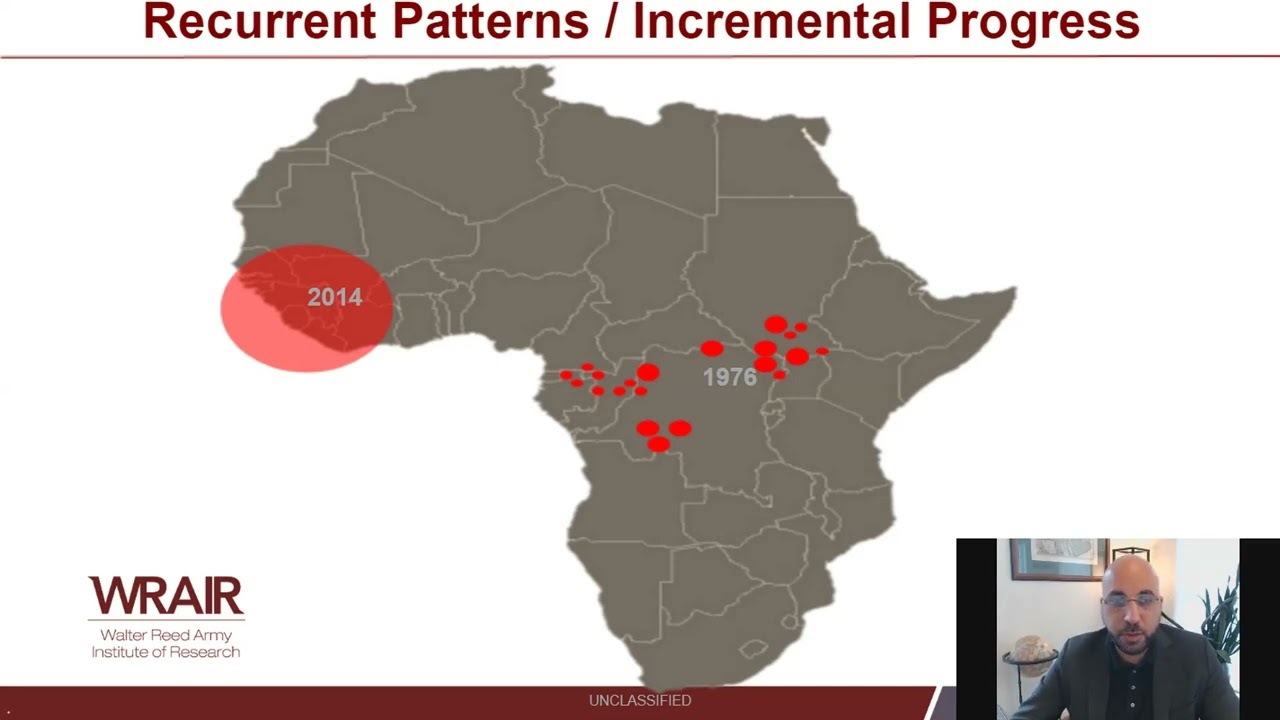
Webinar Abstract: The need for next-generation, broadly protective coronavirus vaccines is underscored by the accelerated pace at which human coronaviruses have been emerging, the evolution of the current pandemic and the long-term threat of a continual rise of novel coronavirus variants, strains and species. In an effort to take a forward-looking approach toward coronavirus vaccine development, we designed and developed an adjuvanted SARS-CoV-2 Spike ferritin nanoparticle (SpFN) vaccine that confers a wide breadth of protective immunity against multiple sarbecovirus lineages. We found that SpFN elicits potent, broadly neutralizing antibody responses against wild-type SARS-CoV-2, variants of concern as well as SARS-CoV-1 that exceed levels elicited by other major vaccines. The robust immunity elicited by this vaccine translates into rapid protection against virus challenge across multiple animal models. With its current evaluation in a first-in-human clinical trial nearing completion and plans for further development, SpFN’s may extend beyond sarbecoviruses to serve as a promising platform as a broadly protective coronavirus vaccine.
Learning Objectives:
1. Describe the basic design and nanoparticle approach of a vaccine that confers broadly protective immunity against sarbecoviruses.
2. Summarize the development pathway of an adjuvanted SARS-CoV-2 Spike ferritin nanoparticle vaccine through preclinical assessements and translation to clinical evaluation.
3. Introduce the key considerations for the clinical development for a broadly protective sarbecovirus and betacoronavirus vaccine in the context of a immunologically primed population.
LabRoots on Social:
Facebook: [ Ссылка ]
Twitter: [ Ссылка ]
LinkedIn: [ Ссылка ]
Instagram: [ Ссылка ]
Pinterest: [ Ссылка ]
SnapChat: labroots_inc




































































![Как работает Клавиатура? [Branch Education на русском]](https://s2.save4k.org/pic/xCiFRXbJTo4/mqdefault.jpg)