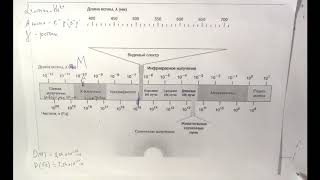
This conference was intended to provide basic instruction in the registration and listing policy and process for those who are new to this regulatory program as well as offer regulatory professionals more in-depth information on issues and current events affecting Drug Registration and Listing.
Timestamps
00:26 – Recent Automated Validation Rules
15:15 – Case Studies
29:28 – Q&A Discussion Panel
Speakers:
Lalnunpuii Huber
Technical Information Specialist
Drug Registration and Listing Branch (DRLB)
Division of Labeling, Registration and Unapproved Drugs (DLRUD)
Office of Unapproved Drugs and Labeling Compliance (OUDLC)
Office of Compliance (OC)
Center for Drug Evaluation and Research (CDER) | FDA
Julian Chun
Pharmacist
DRLB | DLRUD | OUDLC | OC | CDER | FDA
Panelists:
Lalnunpuii Huber, Julian Chun
and
Yajun (Jason) Tu, PharmD, PhD, BCSCP
LCDR, USPHS
Program Management Officer
Policy and Operations Branch (POB)
Division of User Fee Management (DUFM)
Office of Management (OM) | CDER | FDA
David Mazyck
Consumer Safety Officer
DRLB | DLRUD | OUDLC | OC | CDER | FDA
Soo Jin Park
LCDR, USPHS
Regulatory Officer
DRLB | DLRUD | OUDLC | OC | CDER | FDA
Tasneem Hussain
Pharmacist
DRLB | DLRUD | OUDLC | OC | CDER | FDA
Vikas Arora
Pharmacist
DRLB | DLRUD | OUDLC | OC | CDER | FDA
Leyla Rahjou-Esfandiary
Lead Consumer Safety Officer
DRLB | DLRUD | OUDLC | OC | CDER | FDA
Learn more at: [ Ссылка ]
-----------------------
FDA CDER’s Small Business and Industry Assistance (SBIA) educates and provides assistance in understanding the regulatory aspects of human drug products & clinical research.
Upcoming Training - [ Ссылка ]
SBIA Listserv - [ Ссылка ]
SBIA 2022 Playlist - [ Ссылка ]
SBIA LinkedIn - [ Ссылка ]
SBIA Training Resources - [ Ссылка ]
Twitter - [ Ссылка ]
Email - CDERSBIA@fda.hhs.gov
Phone - (301) 796-6707 I (866) 405-5367


























































![[UE5] Эффект сонливости. #ue5 #vfx](https://s2.save4k.org/pic/TUd8viidJhM/mqdefault.jpg)
![Гелертер верят - Развитая цивилизация существовала до появления людей? [Времени не существует]](https://s2.save4k.org/pic/pMxzC99_ZkE/mqdefault.jpg)