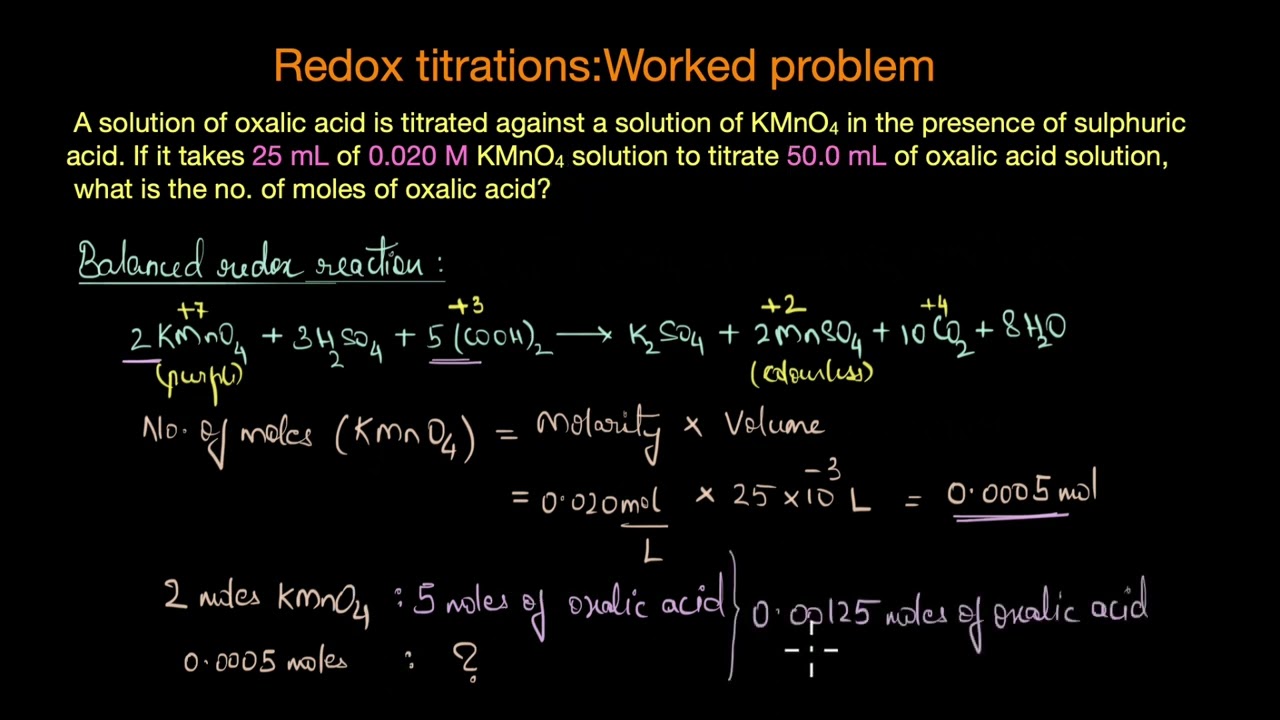
A redox titration uses the principle of the oxidation-reduction reaction where we determine the amount of a substance (also called analyte) by measuring the amount of reagent (titrant) that is required to complete the (redox) reaction. In this video, we discuss one of the most common redox titrations between potassium permanganate and oxalic acid.
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - [ Ссылка ]
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: [ Ссылка ]
Created by
Revathi Ramachandran













![[中文字幕] 唯識三十頌 - 第十九講 - 觀成法師主講](https://i.ytimg.com/vi/UT-6rbimxvg/mqdefault.jpg)