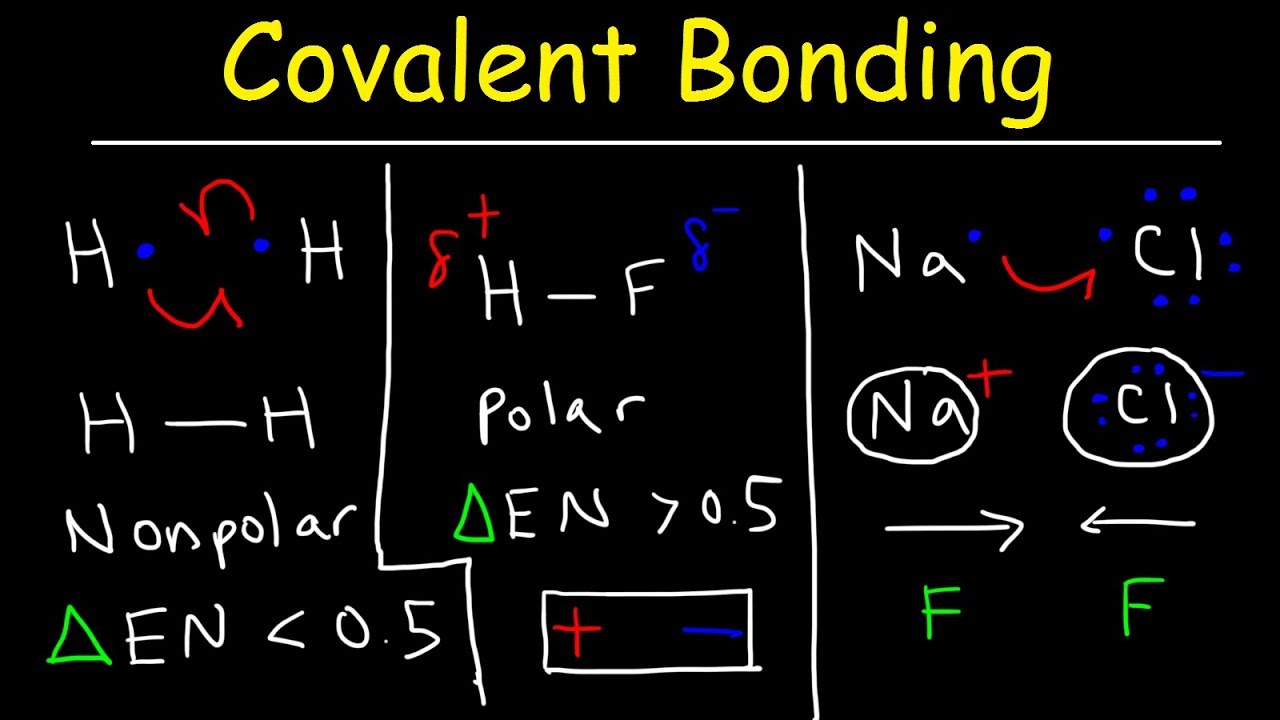
This chemistry video tutorial provides a basic introduction into the types of chemical bonds such as polar covalent bonds, nonpolar covalent bonds and ionic bonds. It discusses the difference between ionic bonding and covalent bonding. Ionic bonds can be identified by looking for a metal combined with a nonmetal. Covalent bonds typically occur among 2 or more nonmetals. Covalent bonding involves a sharing of electrons and ionic bonding forms as a result of a transfer of electrons from the metal to the nonmetal producing ions with opposite charge which are attracted to each other. The electrostatic force of attraction produces the ionic bond that holds the cations and anions together. Polar covalent bonds have unequal sharing of electrons between the atoms where as nonpolar covalent bonding have a relatively equal sharing of electrons between the atoms attached to the bond. Polar covalent bonds typically have an electronegativity difference of 0.5 or more where as nonpolar covalent bonds have a value difference of 0.4 or less.
Ionization Energy:
[ Ссылка ]
Electron Affinity:
[ Ссылка ]
Atomic Radius:
[ Ссылка ]
Bond Energy & Bond Length:
[ Ссылка ]
Electronegativity:
[ Ссылка ]
Periodic Trends:
[ Ссылка ]
__________________________________
Polar & Nonpolar Covalent Bonding:
[ Ссылка ]
Bond Polarity & Dipole Moment:
[ Ссылка ]
Ionic Radius:
[ Ссылка ]
Lattice Energy:
[ Ссылка ]
Born Haber Cycle:
[ Ссылка ]
Bond Energy Calculations:
[ Ссылка ]
___________________________________
Lewis Structures - Mega Review:
[ Ссылка ]
Final Exams and Video Playlists:
[ Ссылка ]
Full-Length Videos and Worksheets:
[ Ссылка ]