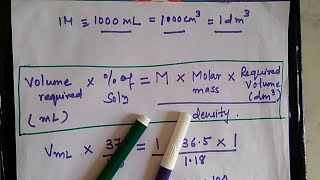Hello everyone,
Standard solution preparation forms the basis of practical chemistry. Here preparation of 1M HCl standard solution is shown. As HCl is available in concentrated liquid form , we have to first convert the molar mass equivalent to volume contained in given % assay in the stock solution i.e. concentrated solution in bottle. So first we shall find out the factor and then calculate required Volume for a given Molar or Normal solution as HCl has same molecular weight and equivalent weight being a monobasic acid.
#1MHClstandardsolution
Happy Learning.