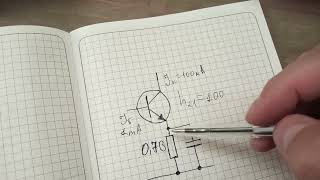
This on-demand webinar hosted by Greenlight Guru explains how to demonstrate conformity to General Safety and Performance Requirements (GSPR) under the Medical Device Regulation (MDR).
This session is designed to help medical device professionals prepare and upgrade their device technical documentation to meet the new requirements.
Main points covered:
1. Differences between essential requirements under the Medical Device Directive (MDD) and GSPR under MDR.
2. Methods to demonstrate compliance to MDR for GSPR.
3. Tips for drafting a GSPR checklist.
4. Understanding the hierarchy of compliance methods, including standards, common specifications, and manufacturer testing.
5. Insights into practical strategies for demonstrating conformity.
Target Audience:
• Regulatory Affairs Professionals & Management
• Quality Professionals and Management
• Medical Device Executives
Access the printable slides for this presentation here:
[ Ссылка ]


![[UE5] Эффект сонливости. #ue5 #vfx](https://s2.save4k.org/pic/TUd8viidJhM/mqdefault.jpg)