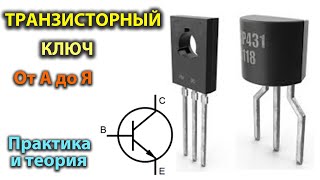
A. Ludi - Results of the 2016 and 2017 proficiency testing schemes for FMD diagnostic methods
Session: Diagnoses & diagnostic tools
Open Session of the EuFMD – 2018 – Increasing Global Security in the supply of effective vaccines – 29-31 October 2018 -Borgo Egnazia, Italy
Introduction
The Pirbright Institute as the OIE, FAO and European Union Reference Laboratory for Footand-Mouth Disease (FMD) carries out an annual proficiency testing scheme (PTS) for laboratories. This exercise is used to demonstrate equivalent performance of diagnostic tests used by FMD International and National Reference laboratories. This presentation summarises the results for the 2016 (Phase XXIX) and 2017 (Phase XXX) exercises and highlights some of the common difficulties that laboratories face in diagnosing FMD.
Materials and Methods
During 2016 and 2017, FMD virological (“live” and inactivated FMDV) and serological panels were made available to laboratories. The particular diagnostic methods that the laboratories utilise were not specified; rather, it was up to each laboratory to select the most appropriate tests using outbreak scenarios that accompanied that samples. For each year, an additional panel was provided to assess whether diagnostic methods can identify the FMDV lineages that are currently circulating. Overall status of each sample was required as well as overall “case” interpretations.
Results
PTS sample panels were sent to 70 laboratories in 2016 and 72 laboratories in 2017. Laboratory performance continues to improve particularly with virological panels; however, technicians continue to encounter serotype cross-reactivity on serological assays and this often leads to mistyping of serum samples.
Discussion
A further PTS for 2018 (Phase XXXI) supported by funding from the EU and FAO/EuFMD is being planned. Please note that we propose to divide the serological panel (Panel 3) into two separate panels: to test (i) outbreak scenarios and (ii) FMDV serotype specificity.
More at [ Ссылка ]




![Futuristic Cities - SCI-FI Designed cities [AI Generated Images] [AI Image Generator]](https://s2.save4k.org/pic/hf-XSeSxdrk/mqdefault.jpg)

















































![Explore the Futuristic Sci-Fi Cities of a distant future | Sci-Fi Futuristic Music [AI Generated 21]](https://s2.save4k.org/pic/n8DbBXzeeyw/mqdefault.jpg)