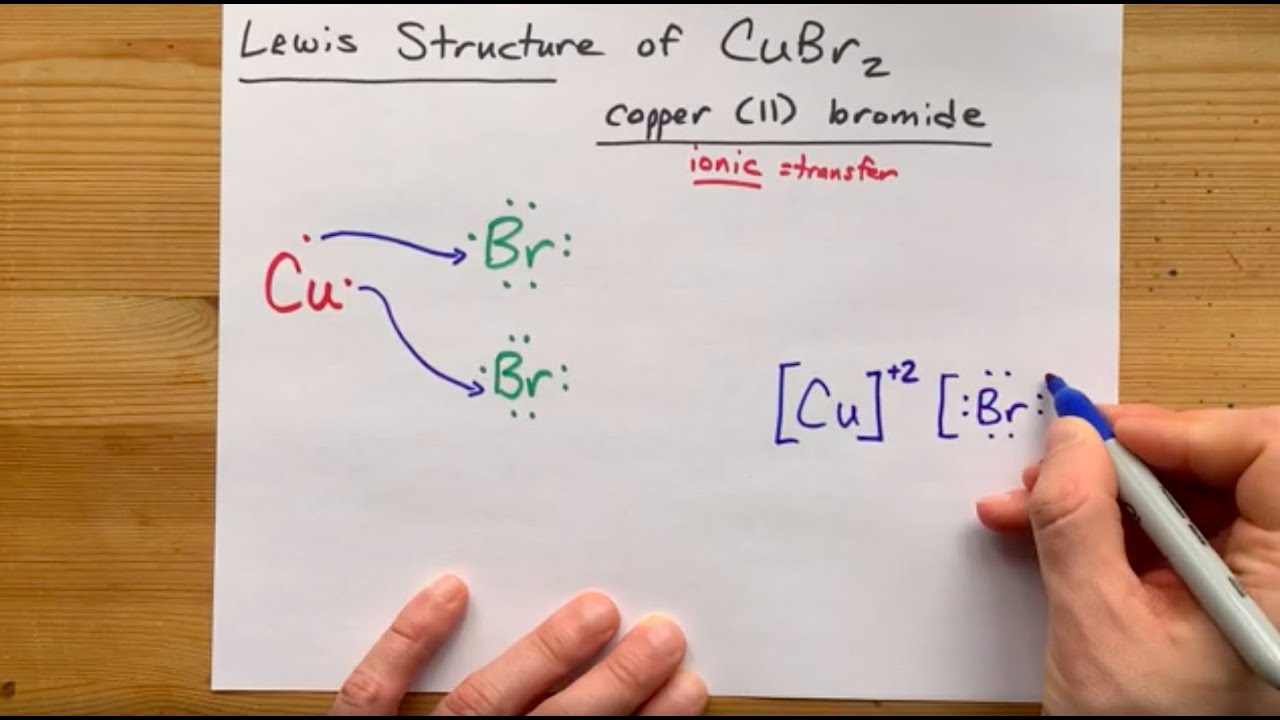
Copper can have two different charges: +1 or +2. In this case, we want the +2 version ... either because of the (II) in the name, or the "2" after the Br in the formula.
So, we draw copper with two valence electrons. It is a metal, so it wants to lose those two electrons.
Bromine is a non-metal which carries seven valence electrons in its outer shell; this is just one short of a full octet.
So a copper atom will give its electrons away to two separate bromines; This completes the octet on the two bromines and leaves copper's "valence shell" empty (I use that term loosely here).
The result is the ionic compound copper (II) bromide, CuBr2. In this video, I draw its Lewis Sturcture including some arrow to show the transfer of electrons.
Check me out: www.chemistnate.com