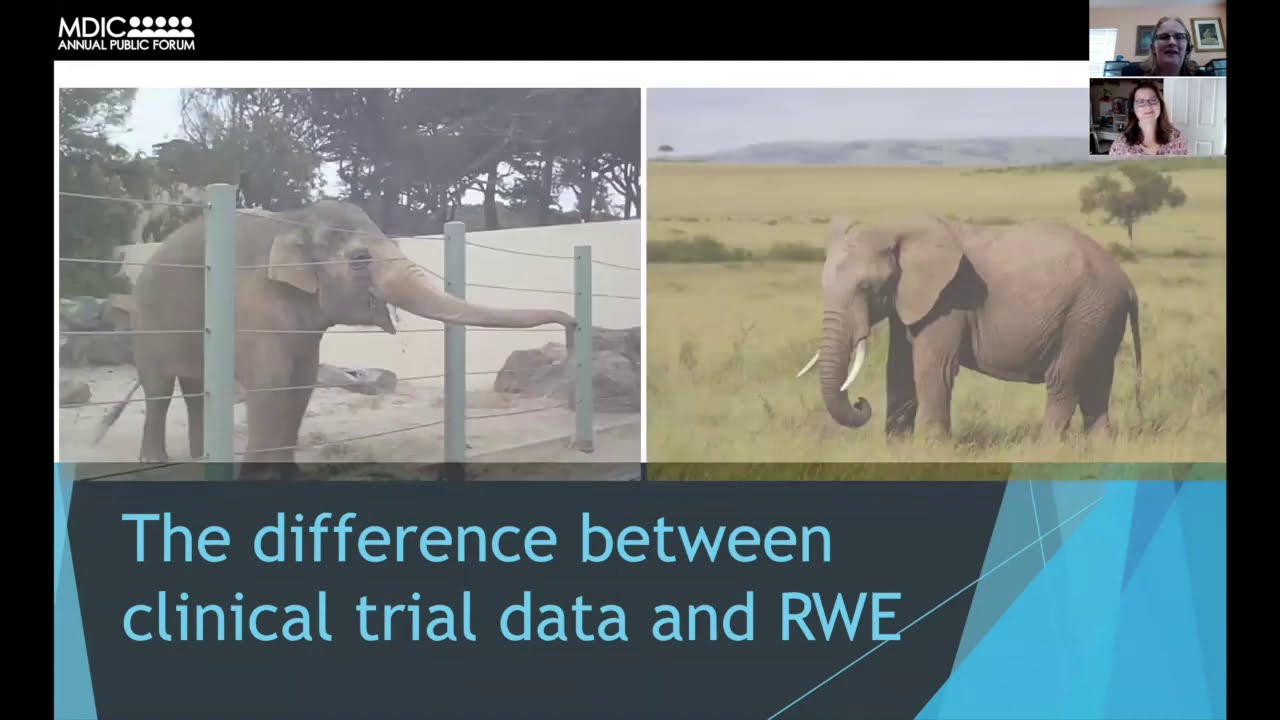
FDA’s CDRH has issued several guidance documents in consideration of using patient preference data in regulatory benefit-risk assessments and real-world evidence to support regulatory decision-making for medical devices. But rather than considering these as two separate topics, device manufacturers should consider these guidance documents together in the development of a cohesive regulatory strategy that maximizes patient input and the potential for regulatory success. Patient preference information can help manufacturers, providers, and regulators understand the level and type of risks patients with a given condition would be willing to endure and the level and type of benefit that would be most meaningful. Real-world evidence has the potential to give a more comprehensive view of how a technology performs and enables manufacturers and regulators to understand the long-term outcomes of medical technology without delaying initial approval. Patient experience and preference information can help inform effective real-world evidence strategies by capturing insight into how patients interact with health technology, their preferences for how their data is used, and their perspectives on risks. A real-world evidence strategy ultimately means working with patients’ real-time and long-term health data and provides an opportunity to capture longitudinal experience and preference information. As such, researchers should seek patient consent to use the data and patient input on how their data are used. Combining the objectives of using patient preference and real-world evidence data in a comprehensive regulatory strategy will ensure sponsors are maximizing the potential for patient input in their regulatory submissions. FDA guidance can be leveraged to ensure this strategy will meet FDA expectations. This panel will discuss the value of patient preference and real-world data and how FDA guidance can be used to ensure a device sponsor’s regulatory strategy meets the needs of both the patient and the regulators.
Speakers:
Stephanie Christopher, MA, CCRC | Senior Program Manager, Health Insights, Providence St. Joseph Health
Carrie M. Kuehn, MA, MPH, LPD, RAC | Evidation Health