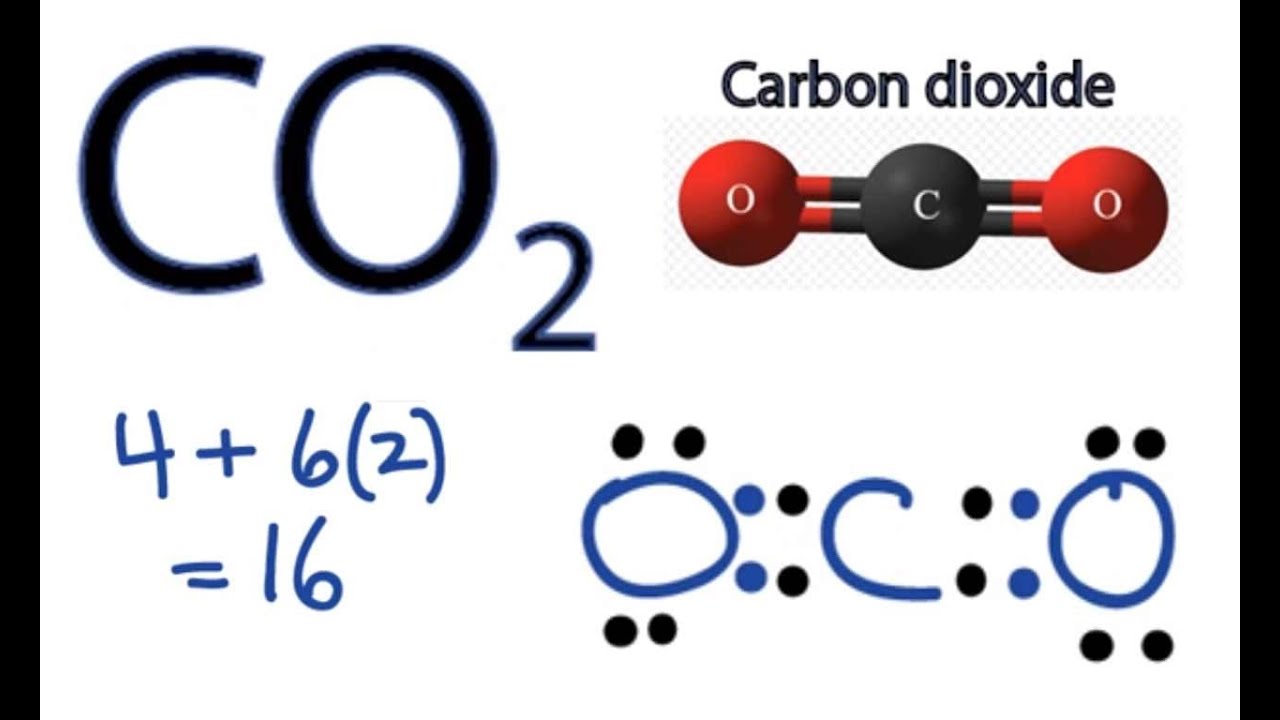A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).
For the CO2 structure use the periodic table to find the total number of valence electrons for the CO2 molecule. Once we know how many valence electrons there are in CO2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure of CO2 structure there are a total of 16 valence electrons. CO2 is also called Carbon dioxide.
----- Steps to Write Lewis Structure for compounds like CO2 -----
1. Find the total valence electrons for the CO2 molecule.
2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside.
3. Put two electrons between atoms to form a chemical bond.
4. Complete octets on outside atoms.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
----- Lewis Resources -----
• Lewis Structures Made Simple: [ Ссылка ]
• More practice: [ Ссылка ]
• Counting Valence Electrons: [ Ссылка ]
• Calculating Formal Charge: [ Ссылка ]
• Exceptions to the Octet Rule: [ Ссылка ]
Lewis Structures are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Carbon dioxide. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule (like boiling point and surface tension).
Chemistry help at [ Ссылка ]