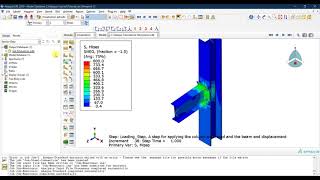
Office of Generic Drugs’ Associate Director for Regulatory Affairs in the Office of Research and Standards Kris Andre shares practical examples on common errors and how to avoid them when using the ePortal to submit a pre-ANDA meeting request.
Learn more at [ Ссылка ]
_______________________________
FDA CDER’s Small Business and Industry Assistance (SBIA) educates and provides assistance in understanding the regulatory aspects of human drug products & clinical research.
Visit [ Ссылка ] and [ Ссылка ] for news and a repository of training activities.
Email: CDERSBIA@fda.hhs.gov
Phone: (301) 796-6707 I (866) 405-5367
LinkedIn: [ Ссылка ]
Twitter: [ Ссылка ]
CDER small business e-mail update subscription: [ Ссылка ]



























































![Как работает Графика в Видеоиграх? [Branch Education на русском]](https://s2.save4k.org/pic/_j8R5vlA0ug/mqdefault.jpg)



![Explore the Futuristic Sci-Fi Cities of a distant future | Sci-Fi Futuristic Music [AI Generated 21]](https://s2.save4k.org/pic/n8DbBXzeeyw/mqdefault.jpg)