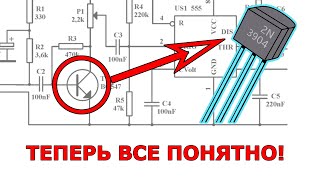
About the educational Session.
On February 20 in 2020 the latest Draft Version of the Annex 1 for the Manufacture of Sterile Medicinal Products was send out to stakeholders like the ISPE for specific comments. The Annex 1 Draft from the European Commission EC is an international supported document where for instance PICs Member states are involved and for that reason the interest of the outcome of the document is of global interest. The Webinar will cover the discussion about Barrier Systems like Isolators. Barrier Systems like Isolators are the first choice from the regulatory authorities to prevent the direct access from the operators to the aseptic critical zone. In the webinar the attendees will learn what is the difference between a Restricted Access Barrier System (RABS) and an Isolator. The aseptic critical zone in an Isolator, air flow and air design requirements in the critical zone , surface decontaminating requirements with vaporized Hydrogen Peroxide vH2O2 and Glove management for Isolator Gloves will be part of the webinar. The word Contamination Control Strategy CCS and Quality Risk Management QRM will be found throughout the whole document from the Annex 1 for Manufacture of Sterile Medicinal Products. The design and qualification of a Barrier System like Isolators for Aseptic Fill and Finish of primary containers of Vials, Syringes etc. are important for an successful pass of an Inspection from the regulatory authorities like the FDA or other regulatory bodies.
About the Presenter
Richard Denk is working at the company SKAN AG, headquartered in Allschwil/Switzerland in the position Senior Consultant Aseptic Processing & Containment. Richard founded 12 years ago the Containment expert group of the ISPE D / A / CH. The Containment Group published the Containment Manual Richard was responsible for in September 2015. Richard is also Chair of the ISPE Special Interest Group Robotic, Steering Committee Member of the CoP SPP Sterile Product Processing, Member of the ISPE European Leadership.



























































![[UE5] Эффект сонливости. #ue5 #vfx](https://s2.save4k.org/pic/TUd8viidJhM/mqdefault.jpg)