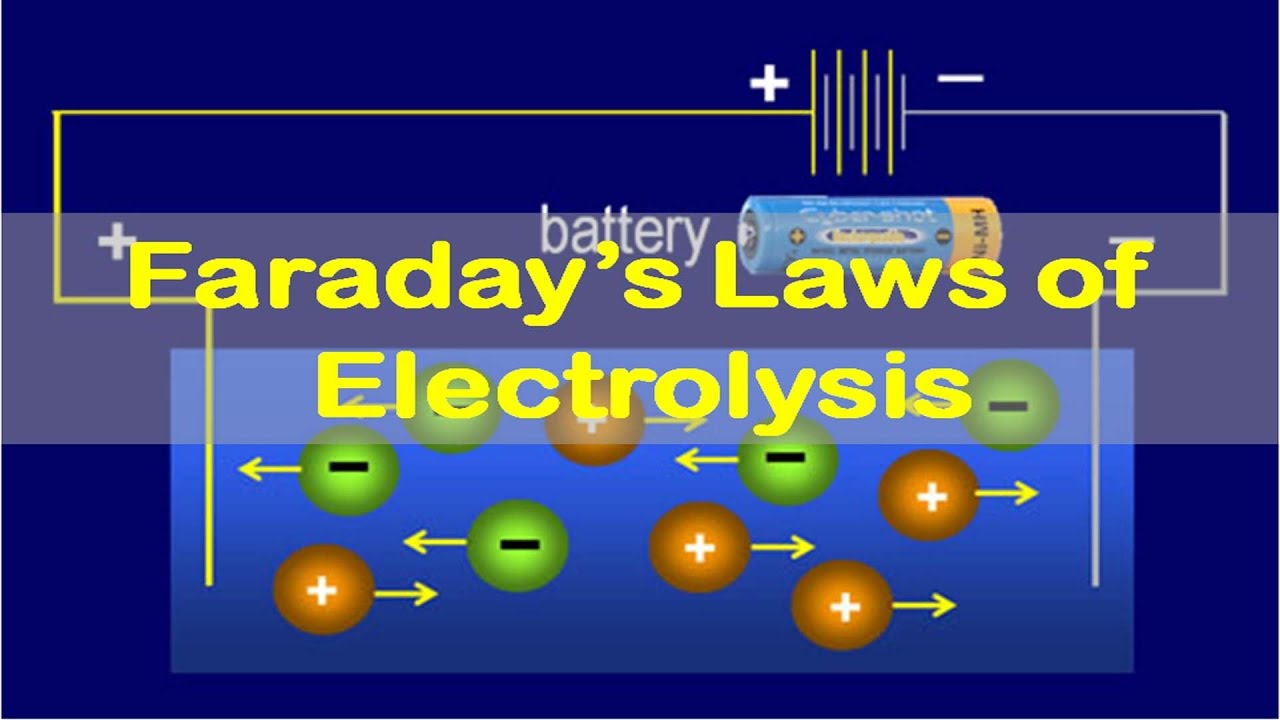
Electrolysis and Electrical Conductance: Faraday Laws of Electrolysis Episode #03
CONTENTS
MECHANISM OF ELECTROLYSIS, ELECTRICAL UNITS, FARADAY’S LAWS OF ELECTROLYSIS, FARADAY’S FIRST LAW, FARADAY’S SECOND LAW, IMPORTANCE OF THE FIRST LAW OF ELECTROLYSIS , MPORTANCE OF THE SECOND LAW OF ELECTROLYSIS, CONDUCTANCE OF ELECTROLYTES, SPECIFIC CONDUCTANCE, EQUIVALENT CONDUCTANCE, SUMMARY OF ELECTROCHEMICAL QUANTITIES, STRONG ELECTROLYTES, WEAK ELECTROLYTES, MEASUREMENT OF ELECTROLYTIC CONDUCTANCE, DETERMINATION OF THE CELL CONSTANT
Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution.[1] For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems.
In many cases, conductivity is linked directly to the total dissolved solids (T.D.S.). High quality deionized water has a conductivity of about 5.5 μS/m, typical drinking water in the range of 5–50 mS/m, while sea water about 5 S/m[2] (i.e., sea water's conductivity is one million times higher than that of deionized water).
Conductivity is traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's Laws of concentration dependence and additivity of ionic contributions. Lars Onsager gave a theoretical explanation of Kohlrausch's law by extending Debye–Hückel theory.
Credits
Essentials of Physical Chemistry by Arun Bahl & J.D Tuli
[ Ссылка ]